Our Approach
Hematologic Malignancies
Our approach in heme malignancies such as acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) is to treat patients following allogeneic bone marrow transplant (hematopoietic cell transplant or HCT) when a patient’s disease burden is at its lowest.
TSC-101 is an HA-2-specific TCR-T therapy candidate directed at eliminating white blood cells, including residual cancer cells, in HLA-A*02:01-positive patients undergoing standard-of-care HCT. HA-2 is a minor histocompatibility antigen that is present on all blood cells, malignant or benign. We believe that healthy donor T cells engineered to express an HA-2-specific TCR will generate an anti-cancer effect in patients, leading to a reduction in relapse rates post-HCT and an increase in long-term survival.
We are currently conducting a Phase 1 clinical study of TSC-101 in patients with AML, ALL, or MDS undergoing standard-of-care HCT. Eligible patients require transplant donors who do not have the HLA-A*02 allele. For more detailed information about our ongoing clinical study please visit: NCT05473910.
Eliminate residual cancer by targeting antigens present on the patient’s, but not the transplant donor’s, blood cells
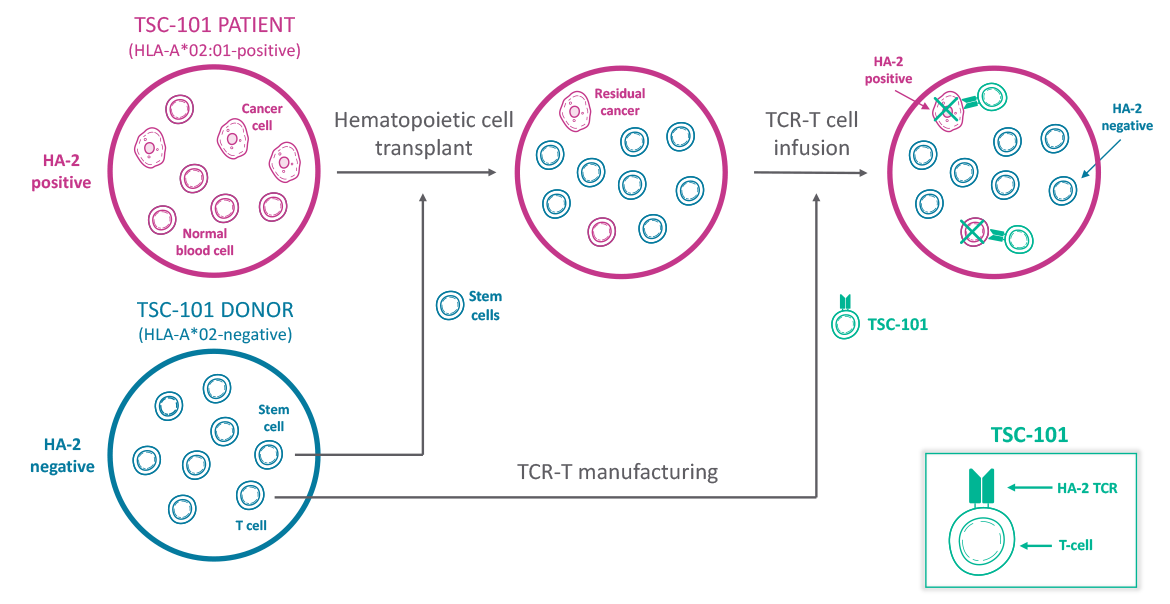
We are focusing on patients that are HLA-A*02:01-positive (as ~97% of patients that are A*02:01 positive are also HA-2-positive) who have donors that are HLA-A*02-negative. While the patient is undergoing HCT, the donor’s T cells are engineered with a TCR that recognizes HA-2. Following the transplant, we infuse the patient with the engineered donor-derived T cells. These TSC-101 cells target any residual cancer cells because the cancer cells are HA-2-positive, but won’t touch any of the new blood cells because they are derived from the donor and are HA-2-negative.
Expansion Opportunities
We plan to expand this program further with the addition of TCRs for additional HLA types. TSC-102-A0301 and TSC-102-A0101 are allogeneic, donor-derived TCR-T therapy candidates targeting epitopes derived from CD45. Like TSC-101, these candidates are designed to eliminate residual cancer cells and prevent relapse in patients undergoing HCT. TSC-102-A0301 and TSC-102-A0101 are designed for patients with HLA types A*03:01 and A*01:01, respectively.