Pipeline
PRODUCT DEVELOPMENT PIPELINE
TScan is leveraging our proprietary platform technologies to develop a robust pipeline of T cell receptor (TCR)-engineered T cell therapies (TCR-T) for the treatment of patients with cancer. Our lead TCR-T candidates, TSC-100 and TSC-101, are in development for the treatment of patients with hematologic malignancies to eliminate residual disease and prevent relapse after allogeneic hematopoietic cell transplantation (NCT05473910). More information about our ongoing clinical trial can be found here.
We are also developing multiplexed TCR-T candidates for the treatment of various solid tumors. Our goal is to build the ImmunoBank, a repository of therapeutic TCRs that recognize diverse targets and are associated with multiple HLA types to provide customized multiplexed TCR-Ts for patients with a variety of solid tumors. In addition, we are applying our platform to identify targets and TCRs in therapeutic areas outside of oncology, such as autoimmune disorders and infectious disease.
(Target)
Hematologic Malignancies
(HA-1)
(HA-2)
(CD45)
(Target)
Solid Tumors (T-Plex)
(HPV16)
(MAGE-C2)
(MAGE-A4)
(PRAME)
(MAGE-A1)
(Target)
Autoimmunity
Hematologic Malignancies Program
- Our hematologic malignancies program is designed to treat patients who are undergoing allogeneic hematopoietic cell transplant (HCT), including AML, MDS, and ALL patients where HCT remains standard of care.
- Despite advances in HCT, as many as 40% of patients relapse following transplant, and relapsing patients face poor prognosis.
- However, some patients naturally develop T cells targeting minor histocompatibility antigens such as HA-1 and HA-2, and these patients exhibit lower relapse rates.
- We are focused on developing TCR-Ts targeting minor antigens discovered in these patients with exceptional responses to HCT-associated immunotherapy, including HA-1 and HA-2.
Our Clinical Development Strategy for Multiple TCR-T Therapies
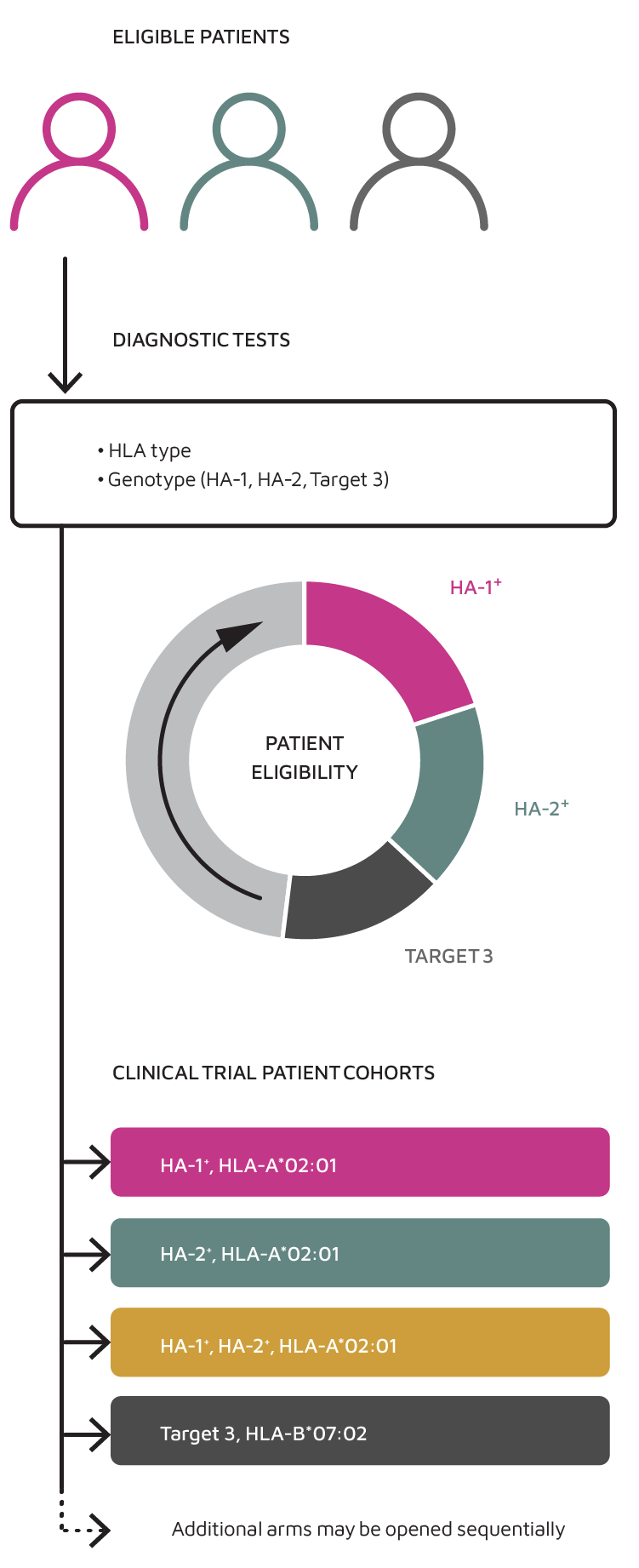
- TScan is leveraging our novel discovery platforms to identify and develop additional minor antigen candidates, expand the addressable patent population for this program, and provide a comprehensive solution for patients.
TSC-100 and TSC-101
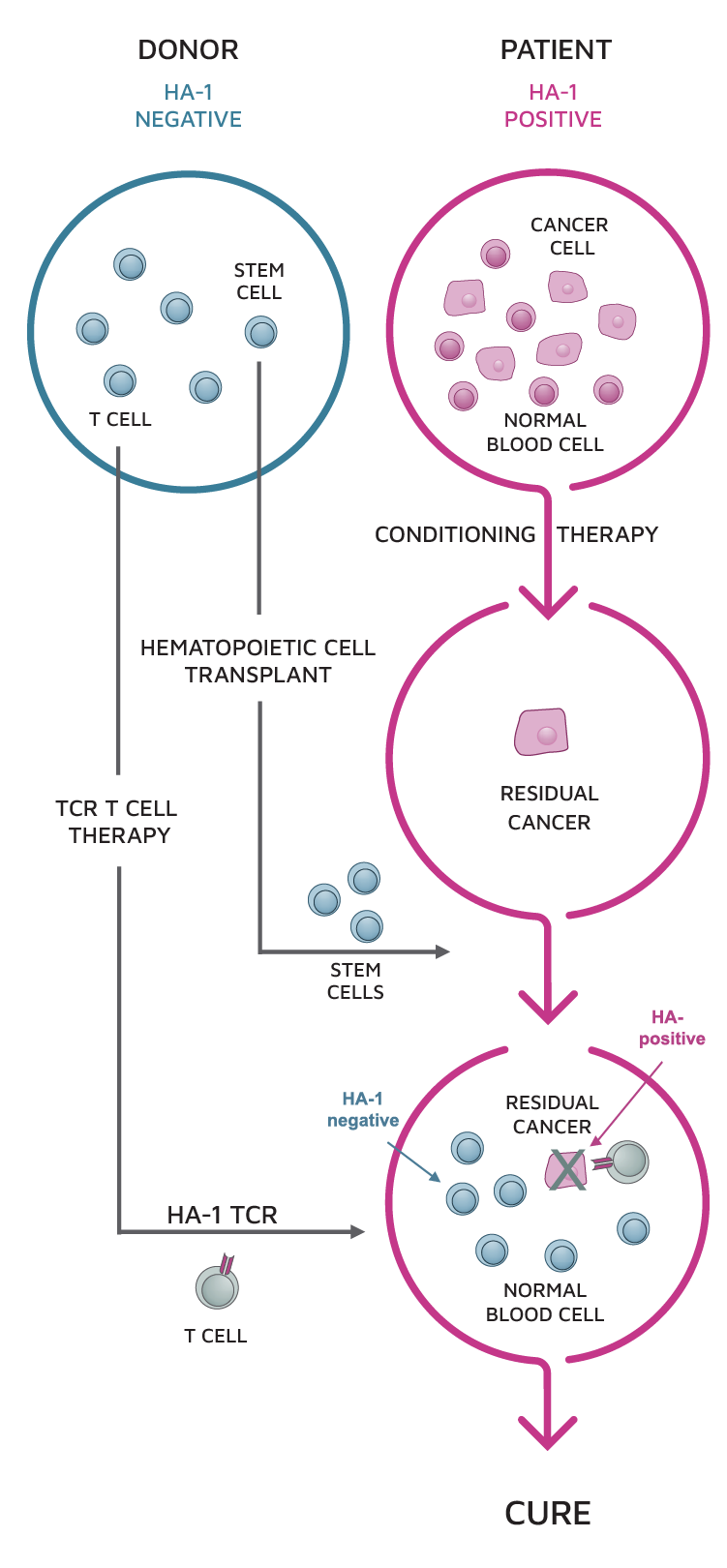
- TSC-100 and TSC-101 are HA-1 and HA-2 specific TCR-T candidates directed at eliminating native blood cells, including residual cancer cells, in target-positive and HLA-A*02:01-positive patients undergoing HCT.
- The TSC-100 and TSC-101 TCRs were identified using ReceptorScan from hundreds of millions of CD8+T cells.
- These therapies are designed to elicit an anti-tumor response in patients by targeting HA-1 or HA-2 minor antigens, which are present on malignant and normal blood cells of patients but not on any of the new, donor-derived blood cells.
- We believe that donor T cells engineered to express an HA-1 or HA-2 specific TCR will generate an anti-tumor effect in patients, leading to a reduction in relapse rates and an increase in long-term survival.
Eliminate residual cancer by targeting blood-specific antigens present in the patient but not the donor
Solid Tumor Program
-
TScan is concurrently developing a multiplexed TCR-T program to treat solid tumors.
-
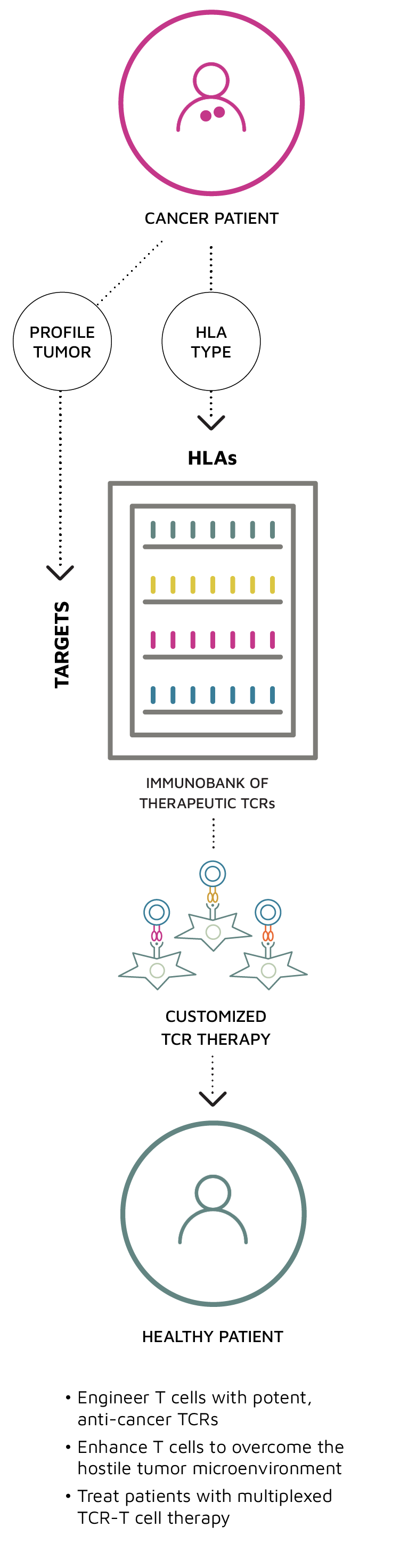
Leveraging our technology platform that enables us to rapidly discover new targets and TCRs directly from the tumor-infiltrating T cells of patients that are actively responding to immunotherapy, we are building a growing ImmunoBank of therapeutically active and safe TCRs with diverse anti-tumor targets.
-
Our vision is that our ImmunoBank will be used to provide tailored therapies for patients with various types of solid tumors.
-
Our TCR-T therapies are designed to be multiplexed to help reduce the risk of resistance driven by target loss, major histocompatibility complex (MHC) downregulation, or mutation of individual HLA genes.
-
Targets are both shared among patients with the same cancer type but are also expressed across different solid tumor types, enabling clinical development in multiple indications.
TScan is building an ImmunoBank of TCRs to enable enhanced, multiplexed TCR-T cell therapy
- Targets are frequently expressed in high incidence solid tumors, including melanoma, head & neck cancer, NSCLC, cervical and ovarian cancer.
- The IND for T-Plex, the primary IND for our solid tumor program enabling customized mixtures of TCR-Ts to be administered to patients based on tumor antigen positivity and HLA expression, has been cleared by the FDA. Our development strategy is to file secondary INDs for each individual TCR-T product under the T-Plex primary IND to ultimately allow for customized multiplexed treatment.
- We plan to combine these products together as a multiplexed TCR-T treatment to overcome tumor heterogeneity and target and HLA loss.
Expansion Opportunities Beyond Oncology
Our primary focus is on the development of T cell therapies to treat cancer. However, T cells play a fundamental role in many other disease areas, such as infectious disease and autoimmune disease. We believe that our TargetScan technology is well suited to discover novel antigens for the development of therapeutics, diagnostics, and vaccines in these other areas. we intend to build additional corporate value by opportunistically pursuing collaborations with strategic partners for applications of our platform technologies outside our core focus.