Technology
SafetyScan
SafetyScan is designed to identify potential off-targets of a TCR and eliminate those TCR candidates that cross-react with proteins expressed at high levels in critical organs.
The ability to identify problematic off-targets is critical as TCR-T therapies engineered with TCRs that recognize off-targets expressed at high levels in critical organs could cause toxicities, thereby limiting their therapeutic potential. We use SafetyScan to screen affinity-enhanced versions of TCRs. Affinity enhancement is a process by which a naturally occurring TCR is mutated in order to generate a more potent therapeutic construct. One such affinity-enhanced TCR that we screened had previously entered clinical trials with a different sponsor, but human testing of the TCR was halted abruptly because two patients treated with T cells engineered to express this affinity-enhanced TCR died of acute cardiac failure within five days of T cell administration. Subsequent studies revealed that this TCR recognized an off-target derived from the muscle protein Titin, which is abundantly expressed in cardiac tissue. When we screened this same affinity-enhanced TCR using our SafetyScan technology, we identified a variety of potential additional off-targets which were not seen in our screen of the natural TCR, including the protein Titin.
Genome-wide screen of affinity-enhanced MAGE-A3 TCR
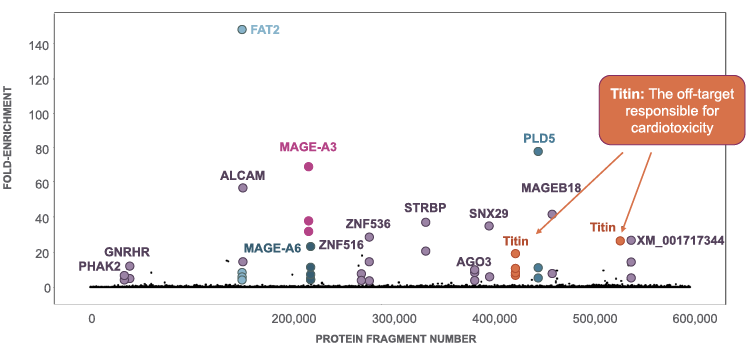
This experiment demonstrates why we believe that our SafetyScan technology provides a significant competitive advantage, because it enables us to rapidly and efficiently eliminate from our preclinical pipeline TCRs that are identified as recognizing potentially problematic off-targets. Importantly, this includes off-targets that may not be identified through standard bioinformatics or in vitro tissue assays. We believe that SafetyScan thereby has the potential to enable us to decrease the risk of encountering unexpected toxicities in our clinical trials by providing a genome-wide understanding of off-target effects.
We believe SafetyScan will allow us to reduce the risk and enhance the potential safety profile of our TCR-T therapy candidates early in development before we initiate clinical trials.