T-Integrate Cell Engineering
Our non-viral manufacturing approach overcomes current constraints of lentiviral delivery, enabling TCR-T multiplexing and T cell enhancements.
Cell therapy manufacturing is highly complex, and associated challenges have led to significant delays or failures in the development of many cell therapies. To enable the rapid, cost-effective, and consistent manufacturing of TCRs, we have developed a non-viral vector delivery system that we refer to as T-Integrate.
Our T-Integrate Manufacturing Platform
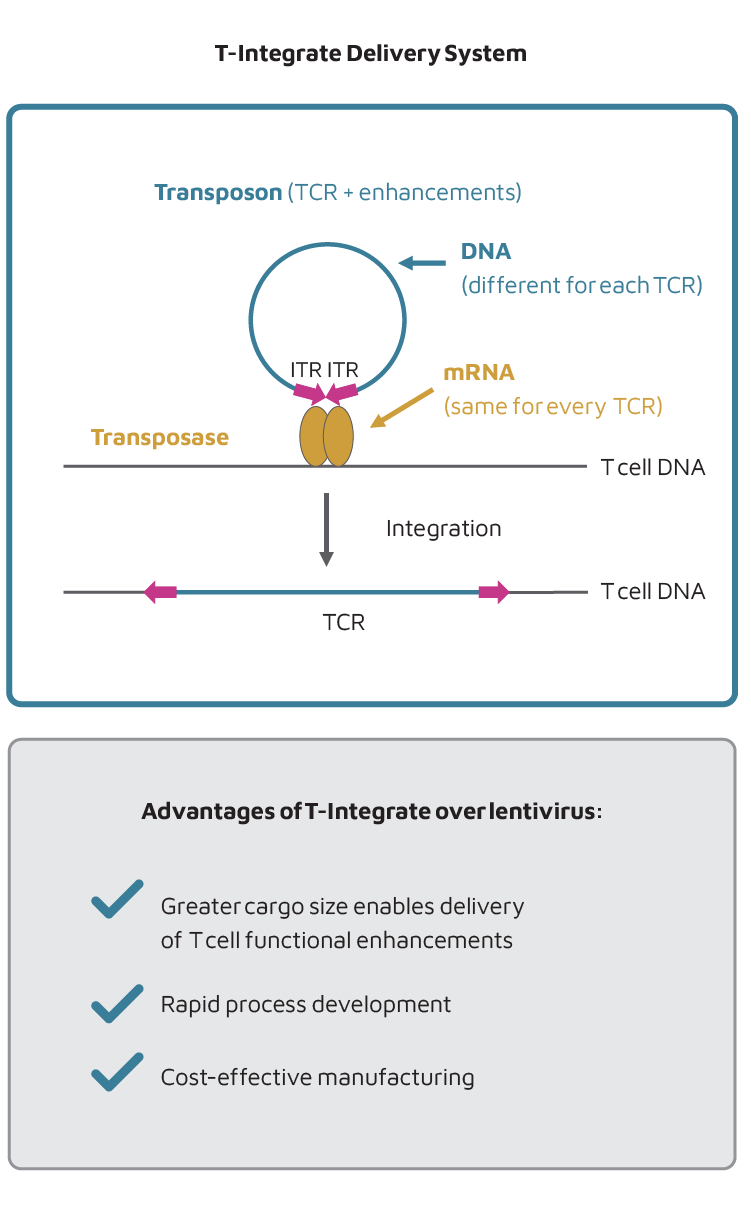
Our TCR-T therapy candidates are manufactured using a transposon/transposase system, in which the DNA encoding the TCR is manufactured as a NanoplasmidTM, a non-viral vector. The Nanoplasmid, together with an mRNA sequence encoding a transposase enzyme, is introduced into the T cell by electroporation. After the T cell translates the mRNA into protein, the transposase enzyme inserts the TCR sequence from the Nanoplasmid into the genome of the T cell.
T-Integrate provides several advantages over lentiviral delivery, including:
- Larger cargo size: Enables delivery of both the TCR as well as functional enhancements to T cells to combat the hostile tumor microenvironment.
- High reproducibility: The only required components for this process are a Nanoplasmid, which is different for each TCR product, and an mRNA, which is constant for all TCR products.
- Cost effective delivery: Transposon system allows for lower cost manufacturing than lentivirus.
- Rapid process development: Lentiviral manufacturing can take significantly longer than DNA manufacturing for T-Integrate.
We believe our manufacturing platform will enable us to efficiently develop and manufacture many different TCR-T therapies, allowing us to deliver customized multiplexed therapy to patients with cancer.